We study few-body processes relevant in chemistry, such as three-body recombination. Our approach is theoretical and numerical. First, we develop new theoretical frameworks capable of dealing with complex chemical reactions in chemical physics, atmospheric physics, and plasma physics. Second, we explore and design new algorithms to reach a proper and efficient simulation of any reaction under consideration.
Ozone formation reaction
Ozone is an essential molecule for the well-being of humanity due to its large absorption properties of UV light in the upper atmosphere. At the same time, ozone in the lower layers of the atmosphere acts as a toxic air pollutant provoking respiratory diseases. It is well established that the oxygen/ozone photochemical cycle governs, to a large extent, the chemistry of the Earth’s atmosphere. Therefore, understanding ozone formation and further ozone-based reactions are crucial for understanding atmospheric dynamics and climate change. This has been an incentive for many studies, including laboratory, ground-based, and satellite measurements. However, many related questions have not yet been answered at the molecular level regarding the theoretical modeling of fundamental three-body interaction processes.
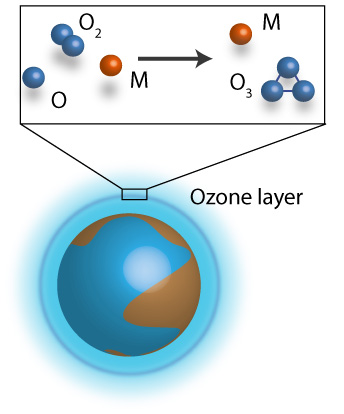
Ozone is formed via ternary reaction O2 + O +M -> O3 + M, where M=Ar, O2, N2. Indeed, these reactions have been observed and measured at various temperature conditions with many efforts to reconcile experimental observations and theoretical interpretations. In theoretical studies, employed the most, although not accurate, is the two-step approach of the ternary reaction. However, in our group we study this reaction from a direct approach using a classical trajectory approach in hyperspherical coordinates allowing a very efficient sampling of the phase space. Our theoretical results show an excellent agreement with the experimental data for temperatures 100-900K. Similarly, our results predict the temperature range in which the traditional proposed mechanisms dominate the dynamics, which generally reconciles different estimates given in previous studies. In addition, it is found that most O3 molecules formed initially (nascent population) are weakly bound and do not survive for a long time in typical experimental conditions. Knowledge of the nascent ozone population is mandatory for reliable interpretation of satellite ozone layer observations in non-local thermodynamic equilibrium conditions, but it cannot easily be obtained from an experiment and must be provided by the theory.
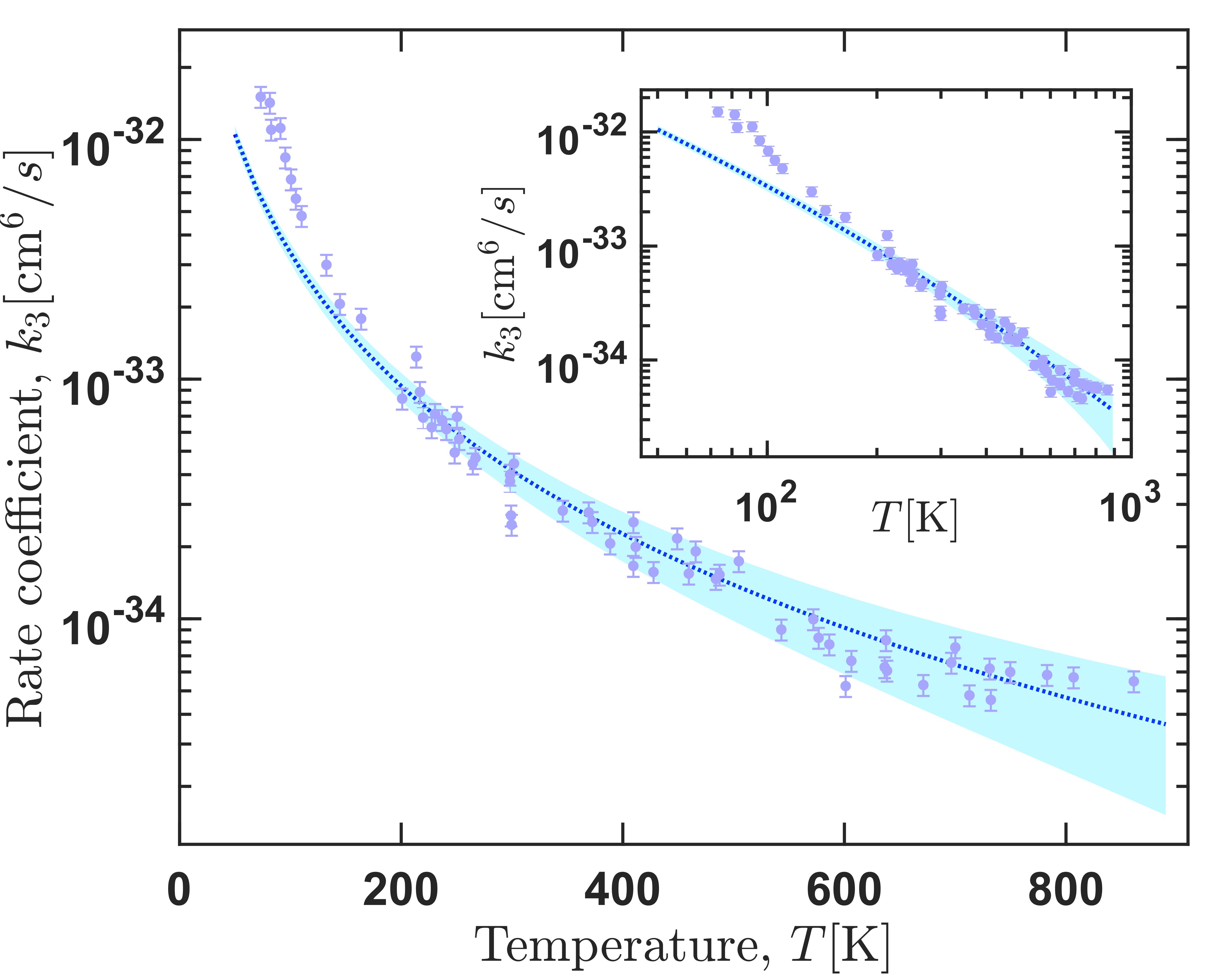
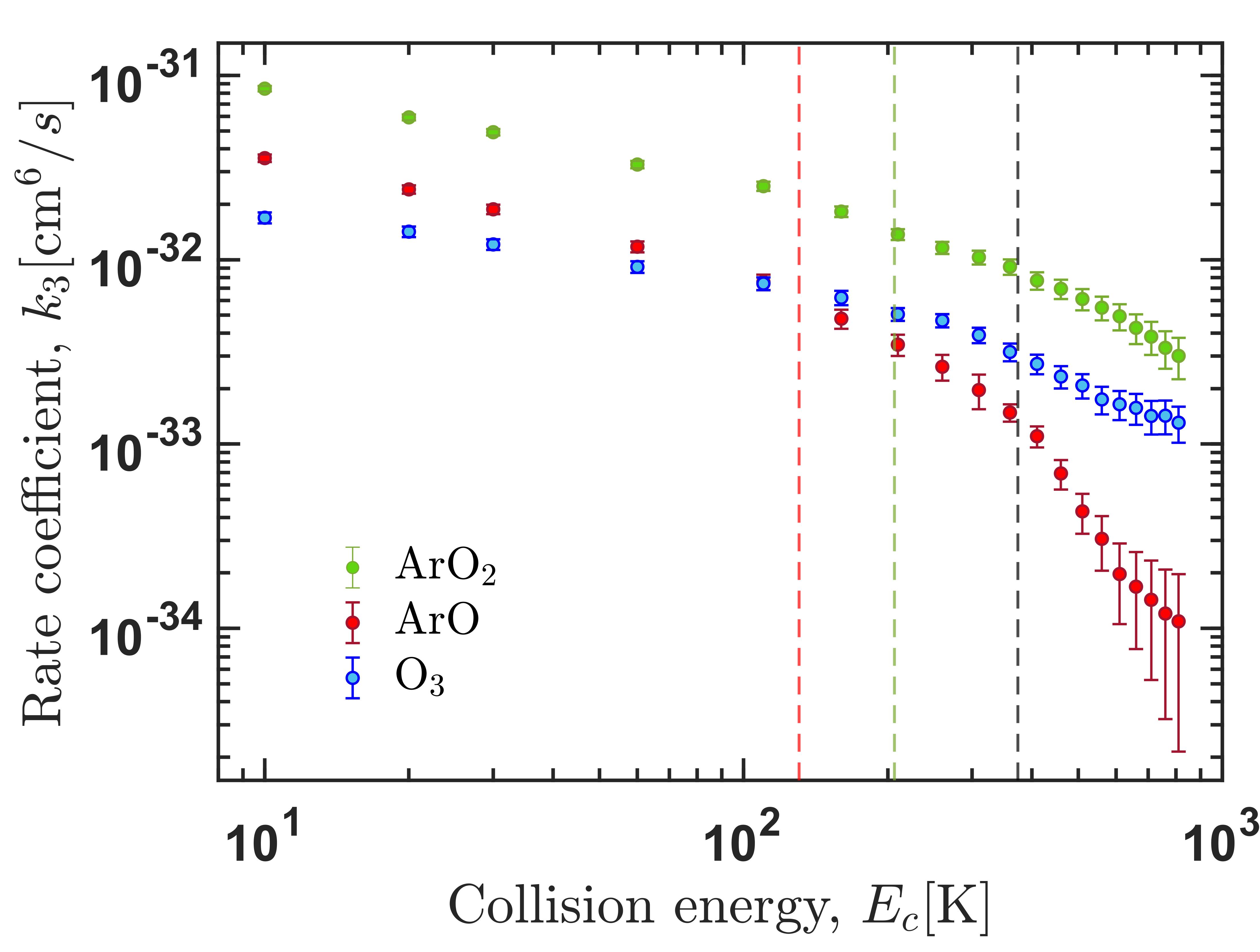
Right panel: Thermally-averaged recombination rate coefficients for formation of ozone accounting for the process of vibrational quenching. The inset shows the same data in the log-log scale. Left panel: ternary recombination rate coefficient for formation of the three possible products.
Van der Waals molecule formation
Van der Waals molecules are unique molecules held by the van der Waals dispersion force. The bonding mechanism appears as a result of the van der Waals force at long-range depending on the polarizability of the atoms and compensating the short-range barrier emerging due to the overlap of closed-shell orbitals. As a result, these molecules show the weakest bound ~ 1meV, despite ultra-long Rydberg molecules. Indeed, van der Waals molecules play a pivotal role in many areas of chemical physics: superfluidity on 4He nanodroplets, chemical reactions, and the stability of liquids, gases, and materials. However, despite the relevance of van der Waals molecules in chemical physics, very little is known about their formation mechanisms. One of them is undoubtedly three-body recombination, i.e., forming a molecule after the collision of three free atoms. However, it has not been extensively studied, barring our work.
He-He-Li collisions leading to an elastic event in panel (a) and formation of LiHe van der Waals molecules formation in panels (b) and (c).
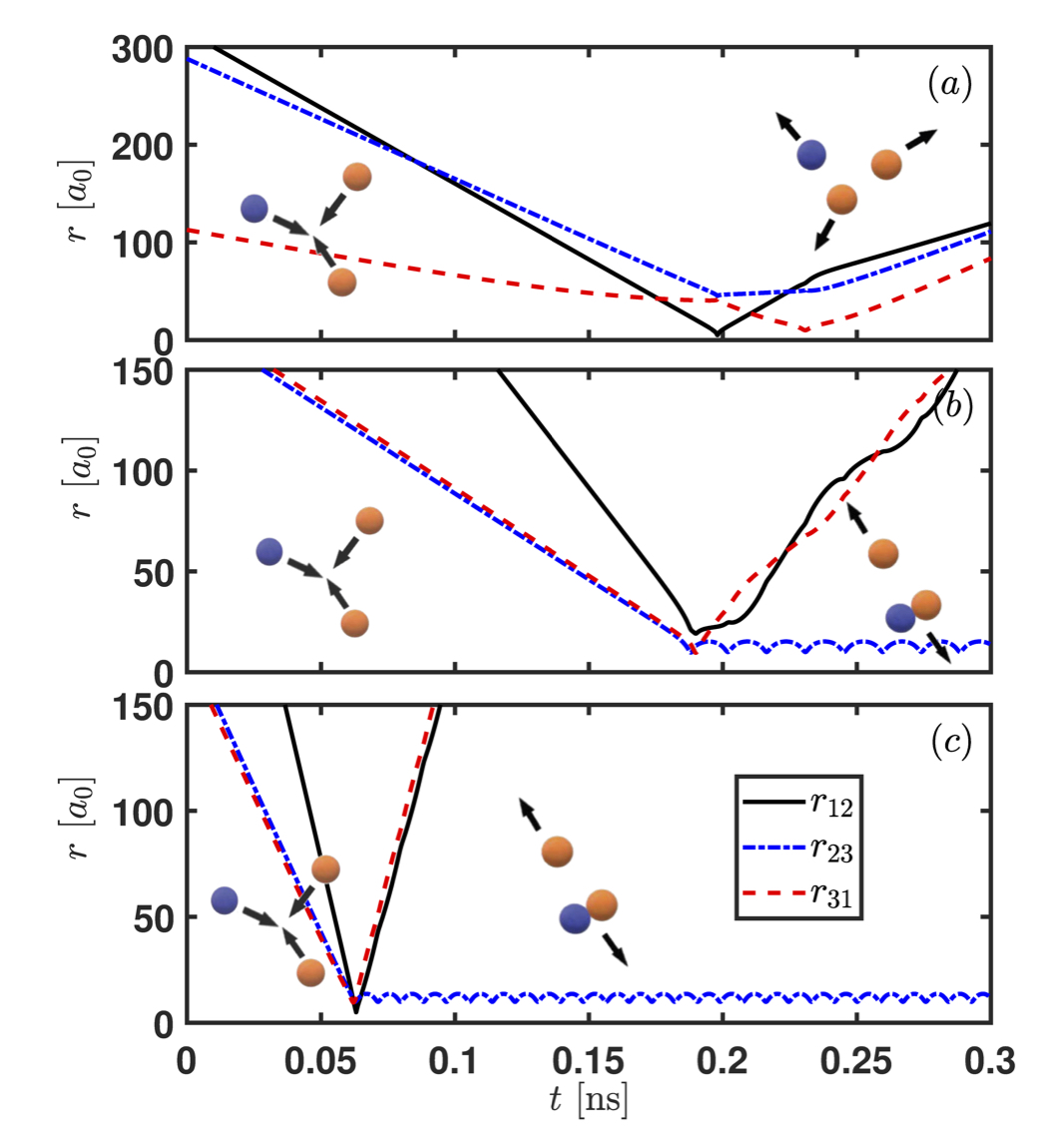
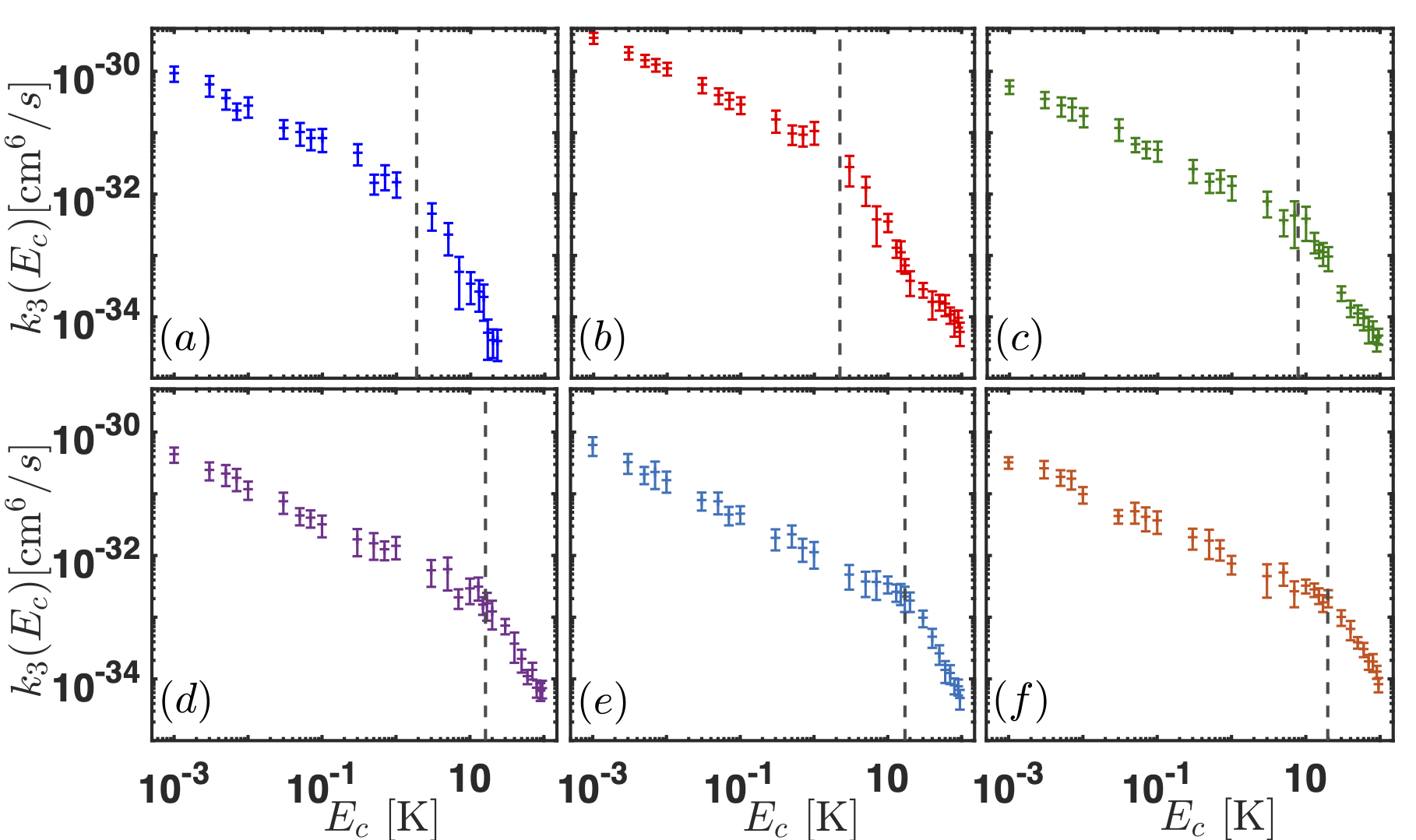
Our group is interested in understanding the origin of van der Waals molecules and how they come to be. To reach that goal, we apply a classical trajectory method in hyperspherical coordinates that have elucidated the formation mechanism of van der Waals molecules in buffer gas cells. Our main result is to realize that the probability of forming van der Waals molecules in a buffer gas cell is independent of the atomic species X. In other words, the reaction rate for the formation of the molecules is the same independently of X, as shown in the Figure below.
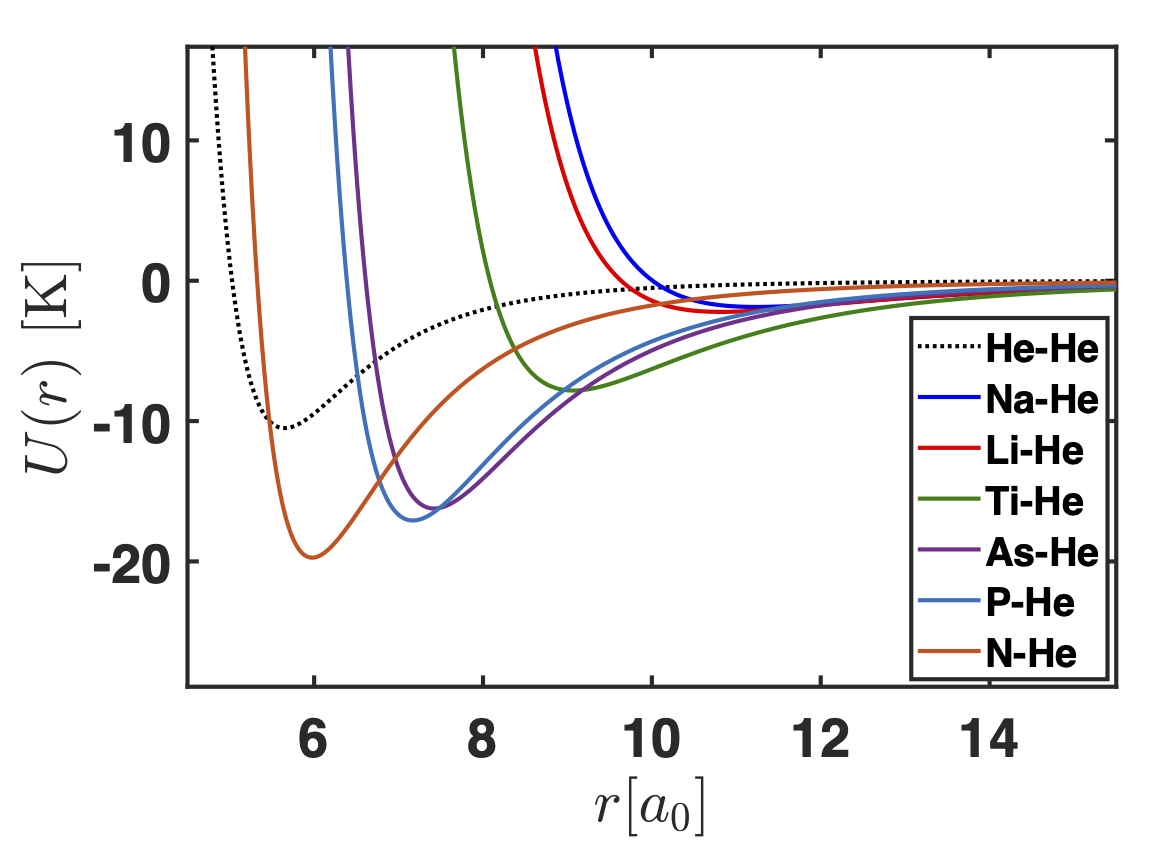
Right panel: Three-body reaction rate for the formation of van der Waals molecules as a function of the collision energy. Left panel: X-He potential energy curves. The color code of both figures is the same: panel (a) corresponds to NaHe, (b) to LiHe, (c) to TiHe, (d) to AsHe, (e) to PHe and (f) to NHe.