Titanate Nanostructures
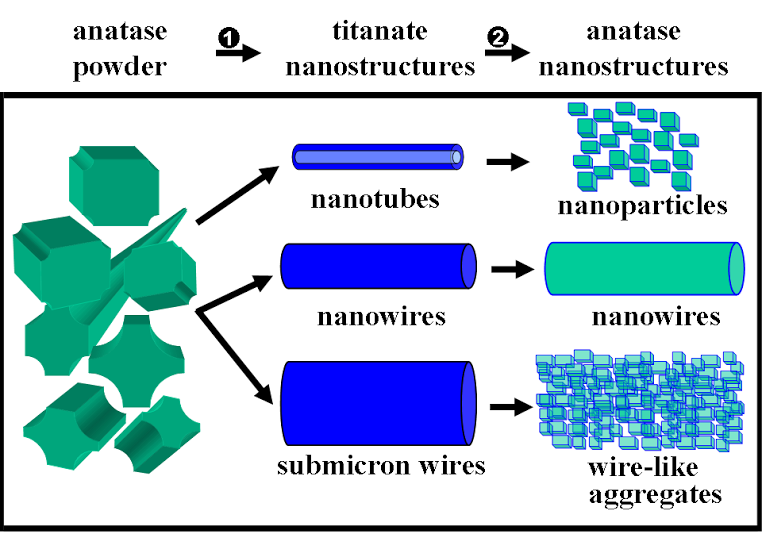
1. A size- and shape-dependent morphological transformation was demonstrated during the hydrothermal soft chemical transformation, in neutral solution, of titanate nanostructures into their anatase titania counterparts. Specifically, protonic lepodocrocite hydrogen titanate nanotubes with diameters of ~10 nm were transformed into exceptionally high-purity anatase nanoparticles with an average size of 12 nm. Lepidocrocite hydrogen titanate nanowires with relatively small diameters (average diameter range of ≤ 200 nm) were converted into single-crystalline anatase nanowires with relatively smooth surfaces. Larger diameter (>200 nm) titanate nanowires were transformed into analogous anatase nanowire motifs, resembling clusters of adjoining anatase nanocrystals with perfectly parallel, oriented fringes.
Refs.: J. Am. Chem. Soc., v.128, 8217 (2006) and Chem. Mater., v.19, 5238 (2007).
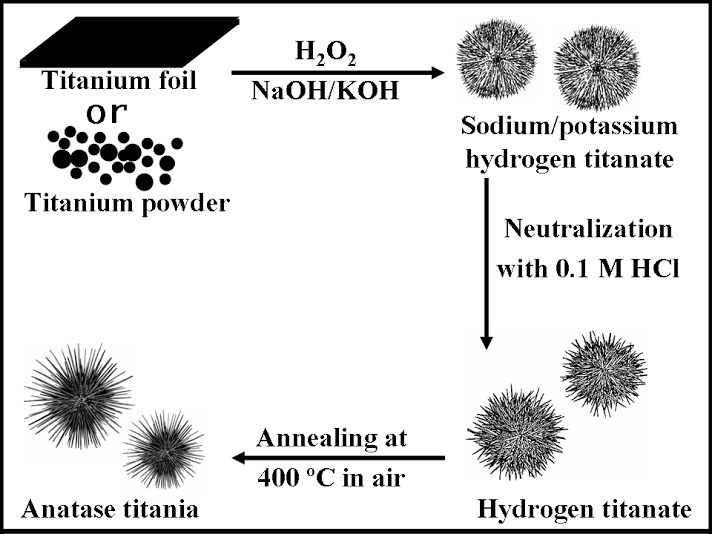
2. Three-dimensional, dendritic micron-scale spheres of alkali metal hydrogen titanate 1D nanostructures (i.e.: nanowires and nanotubes) have been generated using a modified hydrothermal technique in the presence of hydrogen peroxide and an alkali metal hydroxide solution. Sea-urchin-like assemblies of these 1D nanostructures have been transformed into their hydrogen titanate analogues (lepidocrocite HxTi2-x/4 ßx/4O4 (x~0.7, ß: vacancy) by neutralization as well as into their corresponding anatase TiO2 nanostructured counterparts through a moderate high-temperature annealing dehydration process without destroying the 3D hierarchical structural motif. As-prepared hollow spheres of titanate and titania 1D nanostructures have overall diameters, ranging from 0.8 µm to 1.2 µm, while the interior of these aggregates are vacuous with a diameter range of 100 to 200 nm. The constituent, component titanate and TiO2 1D nanostructures have a diameter range of 7 ± 2 nm and lengths of up to several hundred nm. A proposed two-stage growth mechanism of these hollow micron-scale spheres was supported by time-dependent scanning electron microscopy, atomic force microscopy, and inductively coupled plasma-atomic emission spectrometry data. We also demonstrated that these assemblies are active photocatalysts for the degradation of synthetic Procion Red dye under UV light illumination.
Ref.: J. Phys. Chem. B, v.110, 702 (2006).
3. Bismuth titanate (Bi2Ti2O7) nanotubes, with diameters of 180 to 330 nm and lengths varying from 7 to 12 microns, were successfully synthesized using an alumina template-based sol-gel technique. These nanotubes were found to possess higher photocatalytic activity than the corresponding bulk samples prepared without the use of an alumina template.
Ref.: J. Mater. Research, v.21, 2941 (2006).
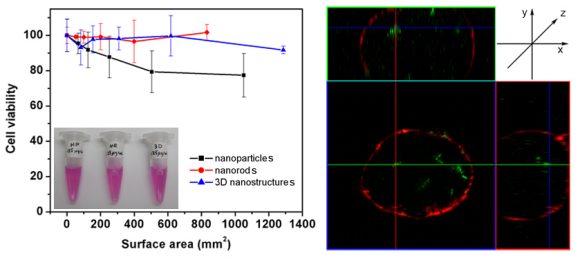
4. We evaluated the cytotoxicity of various morphological classes of TiO2 nanostructures (including 0-D nanoparticles, 1-D nanorods, and 3-D assemblies) to cells. These TiO2 nanostructures were modified with fluorescent dye molecules, mediated via a dopamine linkage, in order to facilitate a confocal study of their internalization. Specifically, we noted that both TiO2 1-D nanorods and 0-D nanoparticles could internalize into cells after 24 h of incubation time. However, only incubation with TiO2 1-D nanorods and 3-D micron-scale sea urchin-like assemblies at concentrations of up to 125 mg/mL yielded data suggestive of cell viabilities of close to 100 %. Moreover, upon irradiation with UV light for periods of a few minutes at energy densities of up to 1 J/cm2, we observed up to 60 % mortality rates, indicative of the cytotoxic potential of photoirradiated TiO2 nanostructures due to the generation of reactive oxygen species.
Ref.: Chem. Res. Toxicol., v.23(5), 871-879 (2010).